MENÜ
AT | EUR
AT | EUR
-
- Tischzentrifugen
- Standzentrifugen
- Gekühlte Zentrifugen
- Mikrozentrifugen
- Mehrzweckzentrifugen
- Hochgeschwindigkeitszentrifugen
- Ultrazentrifugen
- Concentrator
- IVD Produkte
- High-Speed and Ultracentrifuge Consumables
- Zentrifugenröhrchen
- Zentrifugenplatten
- Gerätemanagement
- Proben- und Informationsmanagement
-
- Manuelles Pipettieren & Dispensieren
- Mechanische Pipetten
- Elektronische Pipetten
- Mehrkanalpipetten
- Direktverdrängerpipetten & Dispenser
- Automatisches Pipettieren
- Flaschenaufsatzdispenser
- Pipettierhilfen
- Pipettenspitzen
- Verbrauchsartikel für die Automation
- Zubehör für Dispenser & Pipetten
- Zubehör für die Automation
- Services für Dispenser & Pipetten
Sorry, we couldn't find anything on our website containing your search term.
Multiple targets, one run: multiplex your PCR!
Lab Academy
- Molecular Biology
- Efficiency
- Amplification & PCR
- Quality
- Reproducibility
- PCR Cyclers
- Essay
Cross your heart! What would lab life be without PCR? The “good old” PCR has become second nature to us scientists. If you want to get a variety of information from the same sample, multiplexing makes perfect sense - surprisingly easy without any method change! Raising productivity, saving costs and sample material are only a few of the many advantages. We unravel the secret behind and what to consider…
What the heck was multiplex PCR?
Although we have heard the term a thousand times, there are often uncertainties what is behind it. Maybe you already do multiplex PCR without naming it that way? Here you go: A multiplex PCR amplifies multiple targets within a single PCR run, in a single reaction vessel, from a single sample aliquot - instead of performing many separate reactions. Or, in other words, targeted amplification of multiple genes. You use the same reagent mix, but each target region has its own primer set. Multiplex PCR is used for genotyping, forensics, food testing, pathogen detection, polymorphism analysis and many more applications. It is also part of many targeted sequencing approaches in next generation sequencing (NGS) protocols.
Mehr erfahren
Weniger lesen
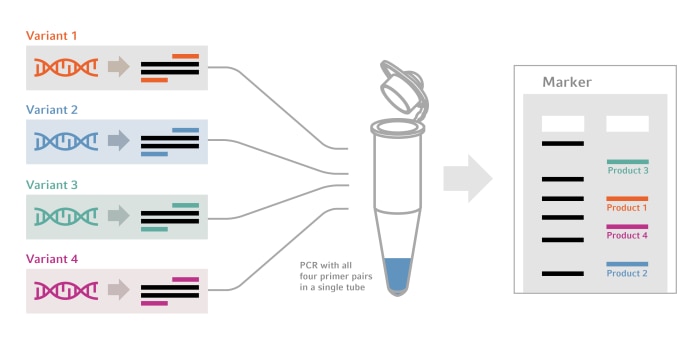
Figure 1: Schematic picture of a multiplex PCR approach.
New benefits with an “old” method
If you stick to singleplexing but wish to get a variety of information from the same sample, you have to perform multiple runs. Multiple runs mean more sample material, more reagents, more time. Accuracy, precision and comparability can suffer, as assays are performed in different vessels with different reaction conditions and with a higher risk of pipetting errors.
One run with various amplicons instead of several runs - the advantages of multiplexing are undoubtedly obvious:
- Increased productivity
- Higher information value
- Saves costs and sample material
- Increased accuracy and comparability
- Improved precision
- No investment in a new cycler and consumables
Putting the cards on the table
Establishing and optimizing the new assay is more complex than a singleplex PCR. Preventing unspecific priming, underrepresented products and loss of yield are key in assay set-up. Typical measures include: primer design with in silico tools, the avoidance of dNTP-depletion ensured by equally abundant templates, optimizing and matching the individual primer combinations with regard to the PCR conditions. Did you know, that thermocycler and consumables are equally important influencing factors?
The impact of thermocycler and consumables
Cycler and consumables can lead to non-specific PCR products, loss of yield and can affect the reproducibility of results. Temperature differences and too high or too low temperatures after heating or cooling ramps (over- and undershoot) can reduce PCR yield and increase the risk of mispriming. Plasticizers, biocides or slip agents in plastic-materials can falsify results.
You can avoid that:
1. Guarantee temperature stability & a low over- and undershoot
Precise ramp rates and stable temperatures avoid unspecific amplifications and allow maximum yields. Likewise, the cycler should control that temperatures are within optimal range, i.e. not too high or too low. Temperature stability and precisely controlled temperatures increase the polymerase’s half-life. The enzyme will thank you with a better yield and drastically reduced misprimed secondary products.
2. Go for leachable-free & clean consumables
Ensure to use “clean” PCR-plates, which are free from leachables, nucleases, DNA or PCR-inhibitors. By the way, thin vessel walls guarantee a perfect temperature transfer. To facilitate that more molecules are available for amplification, use plates where fewer molecules adhere (low binding plates).Knowing pitfalls and the way out, establishing a multiplex PCR protocol is not as complex as you might think!
Did we get you hooked?
Mehr erfahren
Weniger lesen
